Background: For many years, achievement of complete response (CR, currently blasts < 5% blasts by morphology, ANC > 1,000, platelets > 100,000) was the initial goal in acute myeloid leukemia (AML). However, disease recurred in many suggesting residual disease was present. Quantifying such residual disease, and making criteria for CR more stringent (e.g., CR without "measurable residual disease") is an area of ongoing interest. Simultaneously, new responses with criteria less stringent than CR have been promulgated. CR with incomplete count recovery (CRp, CRi) are regularly used with criteria for CR met except either ANC or platelet count does not meet the criteria; in morphologic leukemia-free state (MFLS neither ANC or platelets meet CR criteria). More recently CRh has been suggested. A strict definition of CRh would be as for CR but with ANC 500-1,000 AND platelet 50,000-100,000. A looser definition would allow ANC > 1000 OR platelets > 100,000 but not both (as both would be CR). We note that the strict definition excludes CRi and CRp, while the looser definition would encompass some (but not all) CRi and CRp. While CRh has been used for regulatory approval, its incidence and prognostic impact has not been described. Herein we evaluate these in a recent SWOG Cancer Research Network Phase 3 trial.
Patients and Methods: We analyzed 738 patients from SWOG trial S1203, which randomized adults age < 61 1:1:1 among 7+3, idarubicin + high dose ara-C (IA) and IA + vorinostat. Patients' best responses on protocol therapy were classified using the definitions above. To account for survival after response bias, we performed landmark analyses based on response at day 60; thus, patients who died or were censored before day 60 are excluded from survival analyses. Kaplan-Meier plots were used to estimation survival and Cox regression models were used to assess covariate relationships with overall survival.
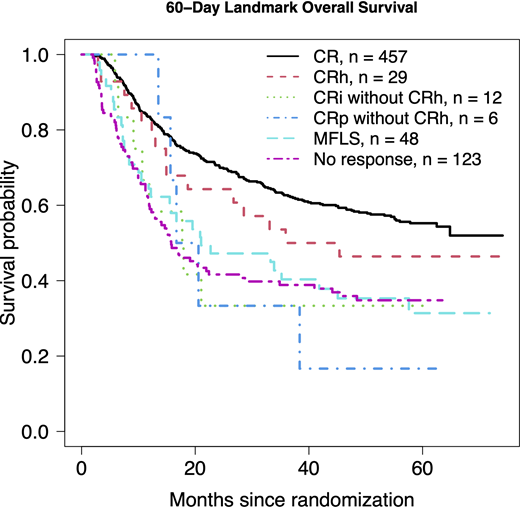
Results: Only 3 patents (0.4%) met the strict criteria for CRh , thus precluding regression analyses. 29 patients (4%) met the looser CRh criteria. Most patients achieved CR, 461 (62%; 457 alive at day 60); 13 achieved CRi without CRh (12 alive at day 60), 7 achieved CRp without CRh (6 alive at day 60), 52 achieved MLFS (48 alive at day 60) and the remaining 176 patients did not achieve a response as defined above (123 alive at day 60). In multivariable Cox regression models controlling age, gender, performance status, WBC, platelets, marrow %, secondary disease, cytogenetic risk, FLT3-ITD, NPM1, and the day-60 variable of one versus two induction cycles, the hazard ratio (HR) for CRh versus CR was 1.12 (95% confidence interval [CI] 0.66-1.91, p=0.67) and versus no response was 0.62 (95% CI 0.36-1.19, p=0.10).
Conclusion: Our data suggest it is plausible a looser definition of CRh and CR have similar beneficial effects on overall survival., but it was also plausible that achieving CRh had a similar association as no response by day 60. But given the small number of patients achieving CRh and the wide confidence intervals in the comparisons, the main conclusion is that more data are needed to understand the effects on survival of CRh vs. other response categories.. We are actively working to collect additional data on AML patients to further understand the role of CRh; we have requested data from trials with patients older than age 60 and patients treated with less-intensive therapies in addition to other trials similar to S1203.
Othus:Marck: Consultancy; Daiichi Sankyo: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees; Glycomimetics: Membership on an entity's Board of Directors or advisory committees. Garcia-Manero:Helsinn Therapeutics: Consultancy, Honoraria, Research Funding; Acceleron Pharmaceuticals: Consultancy, Honoraria; Onconova: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Merck: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amphivena Therapeutics: Research Funding; AbbVie: Honoraria, Research Funding; H3 Biomedicine: Research Funding; Jazz Pharmaceuticals: Consultancy; Celgene: Consultancy, Honoraria, Research Funding; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal